Hello and welcome to the first post here in Physics Notes. In this post, I will be talking about the number of atoms each mole has in a molecule. Although it sounds more like a chemistry topic than physics, moles play a role in physics.
Lets start from the basics. A mole is the measure of the number of discrete particles in a substance, whether liquid, solid or gas. To put it in numbers, 1 mol = 6.023×1023 particles, which is also known as Avogadro’s Number. When we take a mole of a molecule or substance or even compounds, we can tell how many atoms there are in that specific mole or moles.
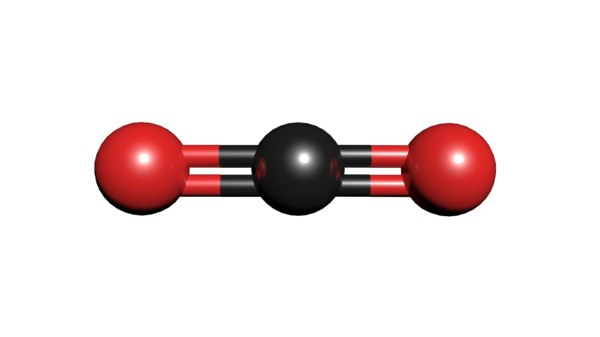
If we take carbon dioxide as an example, we can tell the amount of atoms in 1mol of that molecule by multiplying the number of atoms found in the compound with Avogadro’s number. Ergo, since there is only one carbon atom, we multiply 1 with 6.023×1023 getting 6.023×1023 as the amount of carbon atoms in 1 mol of carbon dioxide. As for oxygen, we multiply 2 with 6.023×1023 to get 1.204×1024 as the amount of oxygen atoms in that mole.
If there is more than one mole of the substance, we first find the amount of atoms for one mole then we multiply the answers with the amount of moles. And that is how atoms and moles are related and number of atoms are calculated.
Until next time, thank you for reading.